Stay Informed
Popular Articles
- Hiatal Hernia: Hidden Cause of Chronic Illness
- Small Intestinal Bacterial Overgrowth (SIBO)
- Applied Lymphology: Unlocking the Secret to Pain Relief
- An Introduction to Constitutional Iridology
- The Low Down on Liver Detoxification
- An Energetic and Emotional Approach to Cancer
- Fat Facts
- Marrow in the Bones
- Blood Type and Nutrition
- Cardiac Herbs: Beyond Hawthorn
Quick Search
The School of Modern Herbal Medicine




Fat Facts
- 7/16/2008
- Categorized in: Nutrition
 This is part one of a four-part article.
This is part one of a four-part article.
I had a lot of unanswered questions and confusion about the whole subject of fats, especially with all the new forms of essential fatty acid supplements that have recently come on the market such as CLA, DHA, etc. So, I did what I always do when I'm having trouble understanding something, I did some research. When I found the information I was looking for, my confusion started to clear, and my next thought was, I'll bet a lot of other people are confused about this issue, too. So I decided to write this article to share what I've learned.
I'll explain the difference between saturated and unsaturated fats, the difference between monunsaturated fats and polyunsaturated fats and the difference between omega-3, omega-6 and omega-9 fatty acids. I'll also explain transfats, conjugated linolic acid (CLA) and other fats and fatty acids, good for us and bad for us. So, if you've been confused about fats, you've come to the right place. Let's start with triglycerides.
Triglycerides
The fats we find in foods are found in the form of triglycerides. You've probably heard of triglycerides because we have them in our blood. So, when you hear someone talk about having a high level of triglycerides on a blood test, that simply means they have a lot of fat in their blood.
![By Wolfgang Schaefer (author) [Public domain], via Wikimedia Commons Triglyceride.PNG](/images/Triglyceride.PNG)
Understanding Saturation
Now, you've probably all heard that there are three basic kinds of fatty acids—saturated, monounsaturated and polyunsaturated. What's interesting is that I got a clearer understanding of this from a cookbook than I did from any of the more “technical” references I looked at. In Alton Brown's book, I'm Just Here for the Food, he compares fatty acids to a line of shoppers, each having two hands which can hold shopping bags. The shoppers are arranged in a long line like you'd find at the checkout counter.
Saturated Fats
In fatty acids, the shoppers are carbon molecules and the shopping bags are hydrogen atoms. So, if all the shoppers in the line have a shopping bag in each hand, the fatty acid would be saturated. There's no more room for any hydrogen atom shopping bags. I don't have the cute illustrations Alton had in his book, but chemically, it would look something like this:
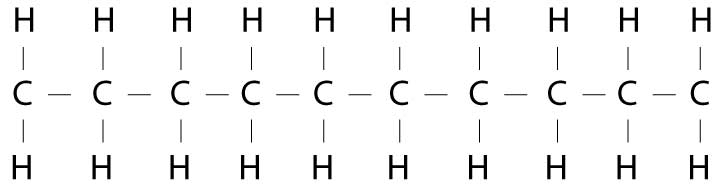
Each of the C's represents a carbon molecule (one of our molecular shoppers). Each carbon molecule shopper is tied to the molecule in front of it and the molecule behind it by a chemical bond (represented by the little lines.) Each carbon molecule shopper has two arms, each of which can hold a molecular shopping bag. This is represented by the H's which are the hydrogen atom shopping bags.
When all the shoppers have a shopping bag in each hand (i.e., all the carbon molecules are holding onto a hydrogen atom) the fat is said to be saturated, because there are no more bonds available for hydrogen atoms.
One completely saturated fatty acid is called steric acid. Steric acid is the primary fatty acid in oils like coconut oil. It is also a primary component of animal fats. A high quantity of steric acid (saturated fatty acids) in any fat will make it solid at room temperature. It also makes the fat more stable in cooking and less likely to go rancid (for reasons we'll explain shortly).
Steric acid makes good fuel for keeping the body warm (which is why Eskimos can get away with eating a lot of saturated fat), but it isn't good for other jobs where the body needs different kinds of fatty acids. See, because they are more solid, saturated fats help to harden cell membranes. This is good up to a point, but if cell membranes get too hard, things have a hard time getting in and out of the cell.
Since the brain is composed primarily of fat, it's good to be a “fat head” because the right kinds of fats make you smart. But, if our diet is too high in saturated fat, then being a “fat head” won't be a good thing because we'll also be “hard headed,” meaning it will be hard for ideas to move in and out of our brain. (I'm only partly joking, because I really believe that the wrong kinds of fats will reduce intelligence, while the right kind will increase it.)
Unsaturated Fats
Continuing with our analogy, our line of shoppers don't like having empty hands. So, when two of the shoppers don't have a bag, they hold hands with each other instead. In chemical terms, this means that when two carbon molecules don't have hydrogen atoms attached to them, they form an additional bond with each other. Chemically, this is called a double bond. Any oil where two of the carbon molecule “shoppers” aren't holding their hydrogen molecule “bag” is unsaturated. Chemically, this looks something like this:
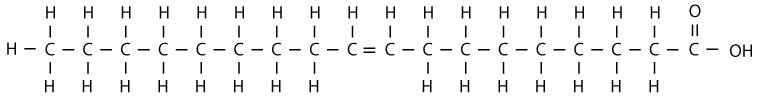
As shown in the above diagram, two of our carbon molecule shoppers don't have hydrogen molecule shopping bags. So, they form a bond with each other instead, which is represented by the double line between them. They are holding hands with each other instead of holding a hydrogen molecule shopping bag. This always happens in pairs (you don't find just one shopper missing a bag).
Monounsaturated Fats
In the above diagram, only one pair of carbon molecule shoppers don't have bags, so there is only one double bond. This makes the fatty acid monounsaturated. (“Mono” meaning one and unsaturated meaning there is room for two more hydrogen atoms.) The primary advantage of monounsaturated fats is their stability for cooking, but they are also liquid, so they won't harden tissues as much. Oleic acid (illustrated above) is a monounsaturated fatty acid which is found in high quantities in olive oil and canola oil, which is why these oils are typically recommended for cooking by nutritionists.
Polyunsaturated Fats
Now, if there is more than one pair missing hydrogen atoms, the oil is polyunsaturated. (Poly means many.) Polyunsaturated fats are liquid at room temperature. In these fatty acids, there is more than one double bond, so more than one pair of shoppers are holding hands rather than holding a shopping bag. Chemically, this looks something like this:
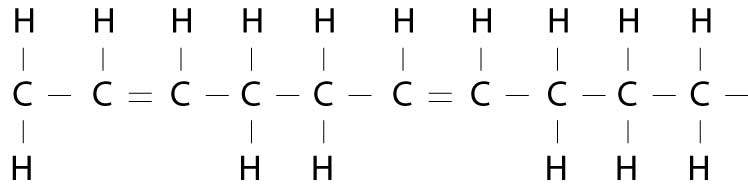
All the fats we eat are actually a mixture of all three types of fatty acids, in varying proportions. For example, coconut oil is 92% saturated fat (i.e., steric acid), 6% monounsaturated fat and 2% polyunsaturated fat. Olive oil, on the other hand, is considered a monounsaturated fat because it is: 15% saturated, 73% monounsaturated (i.e., oleic acid), and 12% polyunsaturated. Safflower oil is a polyunsaturated fat because it is 10% saturated, 13% monounsaturated and 77% polyunsaturated.
Most nutritionists believe that saturated fats are “bad” and monounsaturated and polyunsaturated fats are “good.” Unfortunately, it's not quite that simple. Since all fats contain all three types, the body probably needs some of each. Also, recent research is showing that unrefined coconut oil (even though it is saturated) has many health benefits. So, after doing a little more research I'm not ready to jump on the bandwagon and say, “saturated fats are bad for you, period.” There are other factors.
Essential Fatty Acids
![By Edgar181 (Own work) [Public domain], via Wikimedia Commons Linoleic_acid3D.png](/images/Linoleic_acid3D.png) Here's where I really had a breakthrough in my understanding of fats. All essential fatty acids are polyunsaturated fatty acids. That's why polyunsaturated oils are so important. We need these polyunsaturated essential fatty acids to keep tissues and cell membranes pliable and to perform numerous other functions which saturated and monounsaturated fats can't perform. The reason why polyunsatured fats keep membranes pliable is because they aren't "straight." The unsaturated bonds cause bends in them as shown on the right.
Here's where I really had a breakthrough in my understanding of fats. All essential fatty acids are polyunsaturated fatty acids. That's why polyunsaturated oils are so important. We need these polyunsaturated essential fatty acids to keep tissues and cell membranes pliable and to perform numerous other functions which saturated and monounsaturated fats can't perform. The reason why polyunsatured fats keep membranes pliable is because they aren't "straight." The unsaturated bonds cause bends in them as shown on the right.
But polyunsaturated fats have a problem. Because their carbon molecules aren't saturated with hydrogen, their shoppers have free arms which are available to grab things they shouldn't. That's what turns oils rancid.
As these free arms grab hold of various chemicals in the environment the oil develops an “off” odor and taste. Polyunsaturated oils, having the most free arms, are the most prone to going rancid. And rancid oils don't just taste and smell bad, like any spoiled food, they aren't good for you either.
Hydrogenated Oils and Transfats
The commercial answer to this problem has been the hydrogenation of oils. Hydrogenation simply means that the oil is bombarded with hydrogen in the presence of heat and some chemical reactors, which cause the molecular shoppers who don't have hydrogen shopping bags to pick up a hydrogen molecule.
The problem is that this process adds hydrogen molecules randomly, rather than in the controlled manner nature does. This results in transfatty acids, which look something like this:
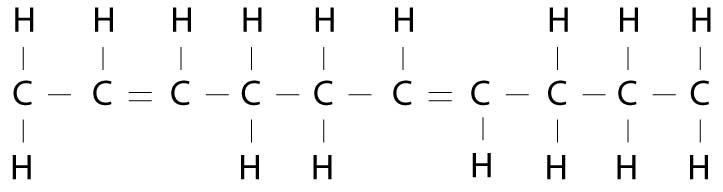
Now, instead of the shopper holding hands on the same side, they have to cross over to the other side. This configuration confuses the body and causes a lot of problems, because it changes the molecular shape of the fatty acid.
Continue reading part two of this series: The Omega Factor
